what does the electron cloud model describe|Electron Cloud : Baguio Learn what an electron cloud is and how it differs from the Bohr model of electrons. An electron cloud is the region of negative . マイページはあなたとpasonaをつなぐ便利なコミュニケーションツールです。mypageには、こちらからログインをしてください。mypageではパソナの最新情報のチェック、おすすめのお仕事の確認や、有給休暇の申請や、給与明細の閲覧など、様々な場面でご利用い .
PH0 · What is the Electron Cloud Model: this is how
PH1 · What Is an Electron Cloud?
PH2 · What Is The Electron Cloud Model?
PH3 · Electron Cloud: Definition, Model, Explanation And Examples
PH4 · Electron Cloud: Definition and Diagram
PH5 · Electron Cloud Theory Explained
PH6 · Electron Cloud Model
PH7 · Electron Cloud
PH8 · Atomic orbital
PH9 · 2.6: Orbitals, Electron Clouds, Probabilities, and Energies
13K Followers, 1,365 Following, 1,961 Posts - Gabrielle (@gabrielle_koster) on Instagram: " Values our ancient connection with the earth and has a heart wish to live accordingly, while navigating through and living in a modern world "
what does the electron cloud model describe*******Home » Pure Sciences. What Is The Electron Cloud? Written by Abhishek Kulkarni Last Updated On: 19 Oct 2023 Published On: 1 Nov 2019. Table of Contents (click to expand) The electron cloud is a cloud of probability surrounding the nucleus in an .
Electron Cloud. In 1913, Niels Bohr proposed the shell model of an atom. He postulated that the subatomic particles, protons, and neutrons reside within the .
The electron cloud is used to describe the behavior of electrons, and it is useful in building a model of the atom. The electron cloud shows the area in space .
Learn what an electron cloud is and how it differs from the Bohr model of electrons. An electron cloud is the region of negative .
At the most basic level, an electron cloud is a region of space around an atom’s nucleus where electrons are likely to be found. This cloud is a fundamental aspect of the way that atoms are.
The solutions to the Schrödinger equation are a set of equations (wave functions) that describe the energies and probabilities of finding electrons in a region of space. They .
Atomic orbitals are the basic building blocks of the atomic orbital model (or electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this .The electron cloud is also defined as the region where an electron forms a three-dimensional standing wave, the one that does not move relative to the atomic nucleus. .The electron cloud model is a model of an atom in which the electrons are attracted to the nucleus by the electromagnetic force and are surrounded by a cloud of rapidly moving electrons. The electron cloud model defines .

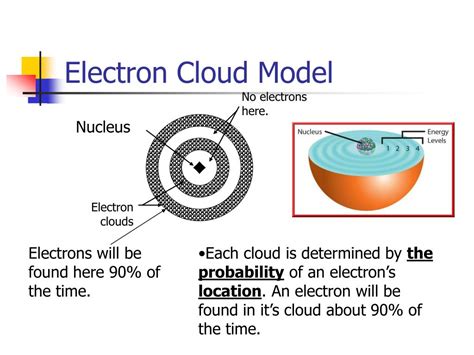
A crash course in electron behavior.—More on the Atomic Model | Wiki—"In quantum mechanics, an atomic orbital is a mathematical function that describes the w. The modern model is also commonly called the electron cloud model. That’s because each orbital around the nucleus of the atom resembles a fuzzy cloud around the nucleus, like the ones shown in the Figure below for a helium atom. The densest area of the cloud is where the electrons have the greatest chances of being. [Figure4]
Electron Cloud The modern model is also commonly called the electron cloud model. That’s because each orbital around the nucleus of the atom resembles a fuzzy cloud around the nucleus, like the ones shown in the . The model is a tool for visualising the most likely electron positions in an atom. The electron cloud model is now the most widely known atom model. Since the electrons were moving so quickly, there was no way of knowing where or when the electron would appear. The electrons were like a blur, which is how they came up with .The electron cloud model defines the zone of probability describing the electron’s location because of the uncertainty principle. The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means . What does the electron cloud model describe? A. the most likely locations of electrons in atoms B. the precise locations of electrons in atoms C. the number of electrons in an atom D. the mass of the electrons in an atom
Erwin Schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. , represents the probability of finding an electron in a given region within the atom. An atomic orbital is defined as the region within an atom that encloses where the electron is likely to be 90% of the time.Figure 22.15 The ground state of a hydrogen atom has a probability cloud describing the position of its electron. The probability of finding the electron is proportional to the darkness of the cloud. The electron can be closer or farther than the Bohr radius, but it is very unlikely to be a great distance from the nucleus. The electron cloud model says that we cannot know exactly where an electron is at any given time, but the electrons are more likely to be in specific areas. Electron cloud model defines the zone of probability describing the electron’s location, because of the uncertainty principle. The electrons in an atom are attracted to the .An atomic orbital, which is distinct from an orbit, is a general region in an atom within which an electron is most probable to reside. The quantum mechanical model specifies the probability of finding an electron in the three-dimensional space around the nucleus and is based on solutions of the Schrödinger equation. The location of the electrons in the quantum mechanical model of the atom is often referred to as an electron cloud. The electron cloud can be thought of in the following way: Imagine placing a square piece of paper on the floor with a dot in the circle representing the nucleus. Now take a marker and drop it onto the paper repeatedly, .
what does the electron cloud model describePreview. 26 terms. Samrmartin14. Preview. Study with Quizlet and memorize flashcards containing terms like What does the electron cloud model describe?, Greek philosopher Democritus coined what word for a tiny piece of matter that cannot be devided?, An electron has a far less mass than either a proton or neutron. True or False? and more.A strength of this model is how it represents the wave behavior of electrons. The fuzzy electron cloud represents how individual electrons are actually spread out through space. Until we measure an electron's position, we don't know exactly where it is. The best we can do is describe where we're likely to find electrons around a nucleus . The electron cloud model describes the distribution of electrons in an atom. According to this model, electrons are not in fixed orbits, as previously thought in the planetary model. Instead, the electron cloud represents the probabilistic locations that an electron may be found around the nucleus. The correct answer to the question is C. 1 Answer. Electron cloud model describes the most probable location of an electron is. It does not say the exact location of the electron as it is moving continuously. Electron cloud model describes the most probable location of an electron is.The electron cloud model is the current model we use to describe atoms. It refers to the cloud of electrons surrounding the nucleus of the atom. Electrons do not travel around the nucleus in simple circular orbits. The location of the electrons in the quantum mechanical model of the atom is often referred to as an electron cloud. The electron cloud can be thought of in the following way: Imagine placing a square piece of paper on the floor with a dot in the circle representing the .
a solid mass surrounded by electrons. What does the electron cloud model describe? rapidly moving electrons. 4.02: Atomic Model. 4.6 (40 reviews) The atomic model that describes an atom as a spherical object containing a certain number of electrons trapped in a mass of positive charge is often called the __________ model.
The performance of HKD to PHP in the last 90 days saw a 90 day high of 7.5458 and a 90 day low of 7.2057. This means the 90 day average was 7.4278. The change for HKD to PHP was -3.64. Track market rates. Compare prices for sending money abroad. Leading competitors have a dirty little secret. They add hidden markups to their exchange rates .
what does the electron cloud model describe|Electron Cloud